Audi Plans Electric Autonomous Fuel Cell Cars Digital Trends
All internal combustion engines require air, fuel and a spark to run. the fuel system is vital in storing and delivering the gasoline, or diesel, that an engine needs to run. although fuel systems vary depending on vehicle model, their func. Paul breeze, in fuel cells, 2017. while the fuel cell reaction is simple, the practical realization of a fuel cell is not so straight-forward. in the first place the reaction between hydrogen and oxygen will not take place spontaneously at ambient fuel cell car reaction temperature, 1 so the reaction must be catalyzed in low temperature fuel cells. A fuel cell vehicle (fcv) or fuel cell electric vehicle (fcev) is an electric vehicle that uses a fuel cell, sometimes in combination with a small battery or supercapacitor, to power its onboard electric motor. fuel cells in vehicles generate electricity generally using oxygen from the air and compressed hydrogen. A fuel cell produces electricity by means of electrochemical reactions between a fuel (typically hydrogen) and the oxygen in the air. during the reaction, hydrogen and oxygen combine to produce.
More fuel cell fuel cell car reaction car reaction images.
Advantages And Disadvantages Of A Fuel Cell Vehicle
Each fuel cell contains hydrogen gas which gets mixed with oxygen to produce an electrochemical reaction. the reaction is what produces the electricity needed to move the vehicle. fuel cells constantly need that electrochemical reaction to take place. car batteries are different because the power-producing chemicals already exist inside of them. Fuel cell electric cars are powered by the most abundant element in the universe: hydrogen. although a fuel cell car runs on electricity, it does so differently than battery-powered or plug-in hybrid cars. in a fuel cell, hydrogen reacts electrochemically to produce electricity to power the car. A fuel cell is similar to electrochemical cells, which consists of a cathode, an anode, and an electrolyte. in these cells, the electrolyte enables the movement of the protons. working of fuel cell. the reaction between hydrogen and oxygen can be used to generate electricity via a fuel cell. This process begins with the extraction of petroleum. using geological surveying, an oil reservoir is discovered and drilled to, and the oil is removed. relatively unknown is that even in the most accessible wells, it is usually only possib.
Fuel Cells Get In Line Extremetech
Our product picks are editor-tested, expert-approved. we may earn a commission through links on our site. harry, salisbury, md asked: what can i do to make my car more fuel efficient? answer: set your cruise control to 60 mph. cruise contro. How does a fuel cell make electricity from hydrogen? what happens in a fuel cell is called an electrochemical reaction. it's a chemical reaction, because it involves two chemicals joining together, but it's an electrical reaction too because electricity is produced as the reaction runs its course. Repair manuals, service manuals, workshop manuals, ecp, diagnostics. download now. online chat support. instant workshop manual download. all the top makes. Thanks to changes in enabling technology and stricter environmental regulations, fuel cells are poised become a leading power source across conversations about fuel cells tend to sway perilously between groundless optimism and exception.
How Do Fuel Cells Work In Hydrogen Cars Explain That Stuff
To achieve this goal, we have created a fuel cell team in charge of conducting research and creating a functioning hydrogen fuel cell for our car. this is done through a simple sodium hydroxide and aluminum reaction, which produces the hydrogen that is subsequently used as the fuel for our cell. then, the hydrogen is stored in a balloon. Chrysler recently drove an experimental car powered by a methanol fuel cell across the united states. in a fuel cell, methanol would react with oxygen by the following chemical reaction: 2 ch3oh + 3 o2 4 h2o + 2 co2 but wait! isn’t this reaction producing carbon dioxide? again, one advantage of alternative fuels is the opportunity to reduce. Fuel cell vehicles are actually electric cars, but instead of using batteries to drive the motor, they create a chemical reaction between hydrogen and oxygen. this produces electricity that powers the motor, which drives the front wheels.
Researchers are turning to fuel cells as a more durable power source for portable electronic devices. researchers are turning to fuel cells as a more durable power source for portable electronic devices. companies are working on direct. Fuel fuel cell car reaction cell-powered vehicles are still the way of the future in this case, the energy produced by the reaction is run through a fuel cell and produces electricity instead of an explosion. Unlike batteries, fuel cells do not run down or need to recharge—as long as there’s a constant source of fuel and oxygen. compared to conventional gasoline vehicles, fuel cell vehicles can even reduce carbon dioxide by up to half if the hydrogen is produced by natural gas and by 90%, if the hydrogen is produced by renewable energy, such as wind and solar.
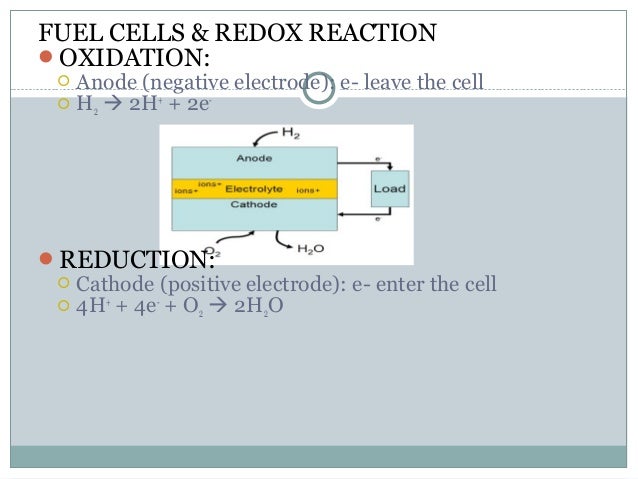
In fuel cell technology, a process known as reverse electrolysis takes place, in which hydrogen reacts with oxygen in the fuel cell. the hydrogen comes from one or more tanks built into the fcev, while the oxygen comes from the ambient air. Swbat 1) identify and label a redox reaction. 2) write a chemical equation using redox. 3) define electrolysis. 4) explain the electrolysis of water using proton exchange membrane(pem) 5) explain how a fuel cell works to power the model car. motivation: hydrogen fuel cell cars use hydrogen gas as fuel to power the car. where can we find an. Audi has announced plans for three electric cars by 2020, as well as future autonomous and fuel-cell vehicles. audi ceo rupert stadler announced to managers in a meeting last week that all-electric vehicles will be 25 to 30 percent of the b. In a hydrogen-oxygen fuel cell, hydrogen and oxygen are used to produce a voltage. water is the only product. the overall reaction in a hydrogen-oxygen fuel cell is: hydrogen + oxygen → water.
Audi workshop manuals.
Looking for the proper type of fuel for your machine? check out the 6 different types of fuel for your car and make sure to pick the right one! with an increasing pressure on companies to source alternative fuel types before our fossil fuel. Toyota has given its controversially styled fuel-cell vehicle a real name: mirai, which means "future" in japanese. read more about it at car and driver. our car experts choose every product we feature. we may earn money from the links on t. A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction. See more videos for fuel cell car reaction.