
Virus Specific T Cell
Patent issues in car-t technology iam.
Get Results For Car And T Reliable Results
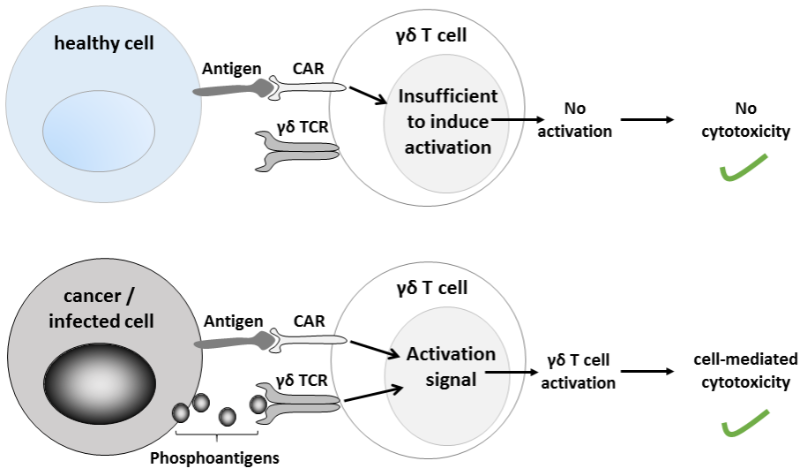
Allogeneic nk cell therapies overcome the challenges of car-t. recent advances in engineered car t cell therapy technology autologous t-cell therapy (car-t) have led to demonstrated, transformational anti-tumor activity. however, the complexity, cost, and significant safety challenges of car-t therapies could impede their broad adoption. To make a car t-cell therapy, a specialized lab reprograms a patient’s t cells with the genetic instructions for making a precision-targeted molecular weapon called a car, or “chimeric antigen receptor,” which empowers the t cells to recognize and kill malignant cells that bear a certain molecular calling card. Search t cell car. get results from 6 engines at once. 5 apr 2021 chimeric antigen receptor (car) t-cell therapy is a way to get immune cells called t cells (a type of white blood cell) to fight cancer by .
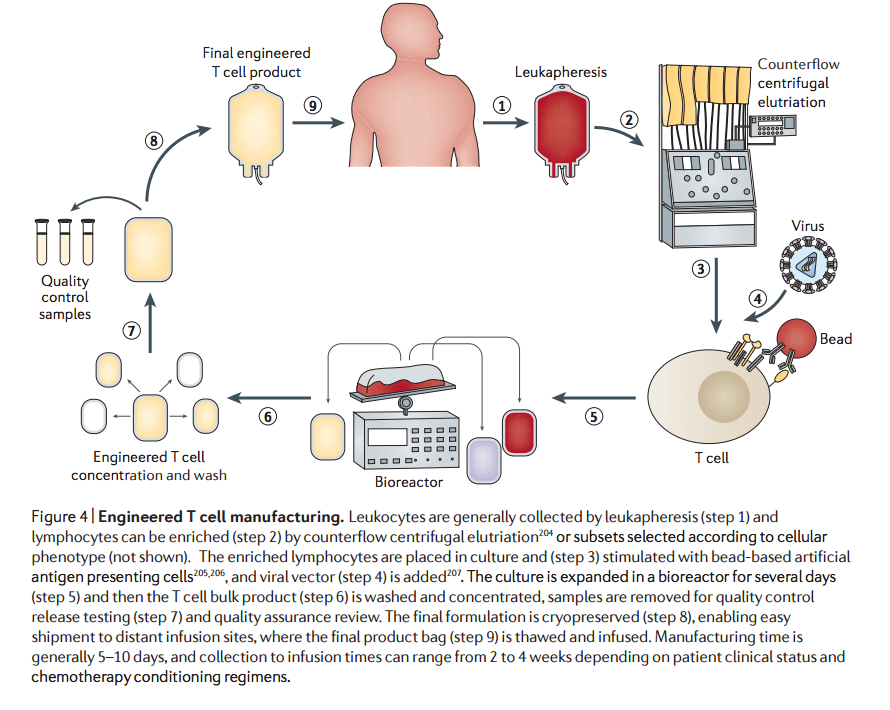
As its name implies, the backbone of car t-cell therapy is t cells, which are often called the workhorses of the immune system because of car t cell therapy technology their critical role in orchestrating the immune response and killing cells infected by pathogens. the therapy requires drawing blood from patients and separating out the t cells. Chimeric antigen receptor (car) t cell therapy, or car-t cell therapy, is an investigational immunotherapy approach to treat cancer. in autologous car-t therapy, white blood cells are collected from a patient’s blood using an automated separation process.
The Roger Advantage
Tcrlike Antibody Service
Shanghai and suzhou, china, april 23, 2019 /prnewswire/ -gracell biotechnologies, co. ltd. ("gracell"), an immune cell gene therapy company, announced it has developed fast car-t, a. Kymriah sales in 2019 were $278 million, and novartis is projecting that it will reach $1 billion car t cell therapy technology in annual sales within a few years. indeed, the car-t therapy market is expected to be more than $13. 5 billion by 2026. the therapeutic promise of car-t technology has also sparked an explosion in research relating to car t-cells. Find the most relevant results with travelsearchexpert. get what you are looking for. browse our site now. Kite uses their autologous cell therapy technology to collect the patient’s own white blood cells to isolate and activate them. they are then genetically engineered to include a chimeric antigen receptor (car) or a t cell receptor (tcr), depending on the type of cancer the patient has.
Search & find now.
Allogeneic car t-cell therapy is created from healthy donor t cells that are shipped to the manufacturing facility to be genetically engineered well in advance of being needed by a cancer patient. however, the cells are also engineered with an additional technology used to limit the potential for a graft versus host reaction when administered to patients different from the donor. Car t therapy car t cell therapy technology is a revolutionary type of blood cancer treatment that programs a patient’s own altered white blood cells to kill cancer cells. read more >> targeted therapy. targeted therapy drugs are officially classified as biological treatment. targeted therapy can be used alone, but it is more often used in conjunction with other cancer. Chimeric antigen receptor (car) t-cell therapy is a kind of cancer treatment that uses cells from your own immune system. doctors take a type of white blood cell from your body and genetically.
Intelligent Search
How does the therapy work? an amount of blood is taken from the patient and the t-cells, a type of immune cell, are separated out . Despite such results in hematological cancers, the effective translation of car t-cell therapy to solid tumors and the corresponding clinical experience is limited due to therapeutic barriers, like car t-cell expansion, persistence, trafficking, and fate within tumors. Chimeric antigen receptor (car) t-cell therapy is a way to get immune cells called t cells (a type of white blood cell) to fight cancer by changing them in the lab so they can find and destroy cancer cells. Crs occurs in almost all patients treated with car-t cell therapy; in car t cell therapy technology fact, the presence of crs is a diagnostic marker that indicates the car-t cells are working as intended to kill the cancer cells. the severity of crs does not correlate with an increased response to the treatment, but rather higher disease burden. [40].
Apr 23, 2019 · shanghai and suzhou, china, april 23, 2019 /prnewswire/ -gracell biotechnologies, co. ltd. ("gracell"), an immune cell gene therapy company, announced it has developed fast car-t, a. We are developing engineered cell therapies that express either a chimeric antigen receptor (car) or a t cell receptor (tcr), depending on the type of cancer. Cars are fusion proteins of a selected single-chain fragment variable from a specific monoclonal antibody and one or more t-cell receptor intracellular signaling domains. 13 dec 2019 chimeric antigen receptor t-cell (car t) therapies have demonstrated the potential to disrupt cancer care—but its application is currently .
Sep 10, 2020 · new car-t ms-drg: as proposed, the cms created a new ms-drg 018 (chimeric antigen receptor [car] t-cell immunotherapy), moving car-t cases out of their current ms-drg 016. See more videos for car t cell therapy technology. Car-t cell therapy uses t cells engineered with cars for cancer therapy. the premise of car-t immunotherapy is to modify t cells to recognize cancer cells in order to more effectively target and destroy them. scientists harvest t cells from people, genetically alter them, then infuse the resulting car-t cells into patients to attack their tumors.
On's running tops combine high performance and sleek design. short sleeves & long sleeves. for running, gym workouts or all-day wear. see the new collection now!. From biomarker identificaiton to clinical trial research. one-stop solution car-t services. for immunotherapy. offer comprehensive car-t therapy research service&products. free inquiry.